1971
Emil Albert Schaerer, inventor of Bodywell® and MobileTek® technology, originally conceptualized the Bodywell® initiative. Schaerer's guiding philosophy was, "My life's mission is to disseminate health worldwide." Inspired by his mentor's commitment, Haim assumed the responsibility of advancing Schaerer's legacy. To this end, Haim initiated further research and development to continue the progress in this critical area of public health.
1980
After a decade of rigorous research and conceptual development, Haim and Emil Albert Schaerer transitioned from theoretical work to practical application by commencing the pre-production phase. Their collaborative efforts aim to actualize their vision, moving one step closer to introducing innovative health solutions to the global market.
2005
Following the initiation of the pre-production phase, prototypes were meticulously crafted and specialized manufacturing facilities were established. This pivotal stage allowed for the commencement of comprehensive product testing, marking a significant milestone in our journey to deliver groundbreaking health solutions to the global community.
2009
Bodywell® USA was formally established, representing a major advancement in our mission to revolutionize global health and wellness. As an integral part of our commitment to quality and efficacy, we have entered the phase of continued evaluation, focusing on rigorous testing of the finalized products. This ensures that we adhere to the highest standards of excellence as we prepare to introduce our innovations to the market.
2011
A pivotal moment in the Bodywell® timeline was the World Health Organization's classification of electromagnetic fields (EMFs) as "possibly carcinogenic to humans." This landmark statement from a globally recognized health authority underscored the urgency and significance of our mission to provide effective mitigation solutions for EMF exposure.
2012
Our products have been rigorously evaluated at the New York College of Technology, focusing on key metrics such as pH levels and torsion fields. These evaluations affirmed the safety and efficacy of our solutions in mitigating the harmful effects of electromagnetic fields and radio frequency radiation.
2012
Dr. Regina Shmelkina conducted an extensive study, utilizing electroencephalograms (EEGs) to further evaluate our products. This specialized research not only adds a layer of scientific rigor to our testing protocols but also provides invaluable data in understanding the product's efficacy and safety metrics.
2013
Bodywell® BioChip becomes available to the public.
2021
Partnered with emater group to help spread the word and awareness and company headquarters opened in ft lauderdale Florida to better service the american market.
2023
Expanding MobileTek® products for new devices and flagship Bodywell® BioCard Pro for enhanced wellness.
Scientific Credibility and Validation
“I can confirm that initial observations indicate either a new scientific breakthrough, an unknown property of the electron or a completely new waveform. The Bodywell® BioChip was initially produced as a detector of some field; it does produce a significant reduction in specific absorption rate in simulated brain tissue indicating that if attached to a cell phone it will reduce the amount of rf-EM energy absorbed by brain cells in proximity of the wireless device.”
“We see that the card application influenced both on alpha-index of EEG and on alpha-power significantly causing in the majority of cases increase of alpha characteristics , in other words, we suggest that the card improves the synchronization of the electrical potentials in the terms of increase of alpha-power and alpha-index.”
“Results clearly indicate that MobileTek® and imprinted SIM considerably prevent variation in pH and both manifest Bio-stabilizing properties.”
Over 10 Years Of Research
Founder, Haim Einhorn has invested more than 10 years and millions of dollars on his quest to create a solution for the potential dangers of RF absorption.
As a worried parent and grandparent, the Bodywell® BioChip was created to provide a layer of protection and peace of mind for safely using RF emitting devices, especially for children.
Haim’s mentor of promoting human health, Emil Albert Schaerer, founded Bodywell® Switzerland in 1971 and his motto was “My life’s mission is to spread health with the world”. Haim took it upon himself to continue his legacy and made it his own life’s mission as well.
Made In Switzerland
The Bodywell® BioChip precise components are all develped and manufactured in the highest quality facilities in Switzerland.
Similar to a high-end timepiece, that requires an extraordinary level of precision, the Bodywell® BioChip is carefully produced and tested to consistently provide effective results.
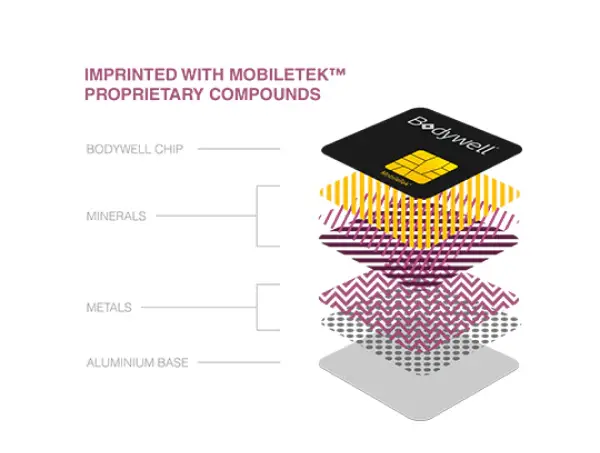
Proprietary MobileTek® Technology
The Bodywell® BioChip has been tested and proven numerous times, in certified labs across the world, to significantly reduce the absorption of radiation by RF emitting devices.
We are extremely proud of our trade secret technology. It is the only technology in the world that has shown consistent reduction of radiation absorption.
<div class="dynamic-checkout__content" id="dynamic-checkout-cart" data-shopify="dynamic-checkout-cart"> <shopify-accelerated-checkout-cart wallet-configs="[{"supports_subs":true,"supports_def_opts":false,"name":"shop_pay","wallet_params":{"shopId":60631875749,"merchantName":"Bodywell","personalized":true}},{"supports_subs":false,"supports_def_opts":false,"name":"paypal","wallet_params":{"shopId":60631875749,"countryCode":"US","merchantName":"Bodywell","phoneRequired":true,"companyRequired":false,"shippingType":"shipping","shopifyPaymentsEnabled":true,"hasManagedSellingPlanState":null,"requiresBillingAgreement":false,"merchantId":"6LBNNKYPPHW52","sdkUrl":"https://www.paypal.com/sdk/js?components=buttons\u0026commit=false\u0026currency=AUD\u0026locale=en_US\u0026client-id=AfUEYT7nO4BwZQERn9Vym5TbHAG08ptiKa9gm8OARBYgoqiAJIjllRjeIMI4g294KAH1JdTnkzubt1fr\u0026merchant-id=6LBNNKYPPHW52\u0026intent=authorize"}}]" access-token="aca3966b8c38a48791f96e65876443ac" buyer-country="AU" buyer-locale="en" buyer-currency="AUD" shop-id="60631875749" cart-id="1993d66f357e442f0715cea24c2fb66f" enabled-flags="["d6d12da0","ae0f5bf6"]" > <div class="wallet-button-wrapper"> <ul class='wallet-cart-grid wallet-cart-grid--skeleton' role="list" data-shopify-buttoncontainer="true"> <li data-testid='grid-cell' class='wallet-cart-button-container'><div class='wallet-cart-button wallet-cart-button__skeleton' role='button' disabled aria-hidden='true'> </div></li><li data-testid='grid-cell' class='wallet-cart-button-container'><div class='wallet-cart-button wallet-cart-button__skeleton' role='button' disabled aria-hidden='true'> </div></li> </ul> </div> </shopify-accelerated-checkout-cart> <small id="shopify-buyer-consent" class="hidden" aria-hidden="true" data-consent-type="subscription"> One or more of the items in your cart is a recurring or deferred purchase. By continuing, I agree to the <span id="shopify-subscription-policy-button">cancellation policy</span> and authorize you to charge my payment method at the prices, frequency and dates listed on this page until my order is fulfilled or I cancel, if permitted. </small> </div>